Track biopharma regulatory updates with Clinicaltrials.gov feeds – Feedly Blog
[ad_1]
Clinical trial updates produce overwhelming amounts of information every day. But a small portion of these articles refers to the diseases and studies you want to monitor.
Cut through the noise and build custom RSS feeds on ClinicalTrials.gov with the information you need.
Go to ClinicalTrials.gov
You are the head of an AIDS research program in a large pharma company. You can create a query on ClinicalTrials.gov to look into clinical trial news coming from other research labs.
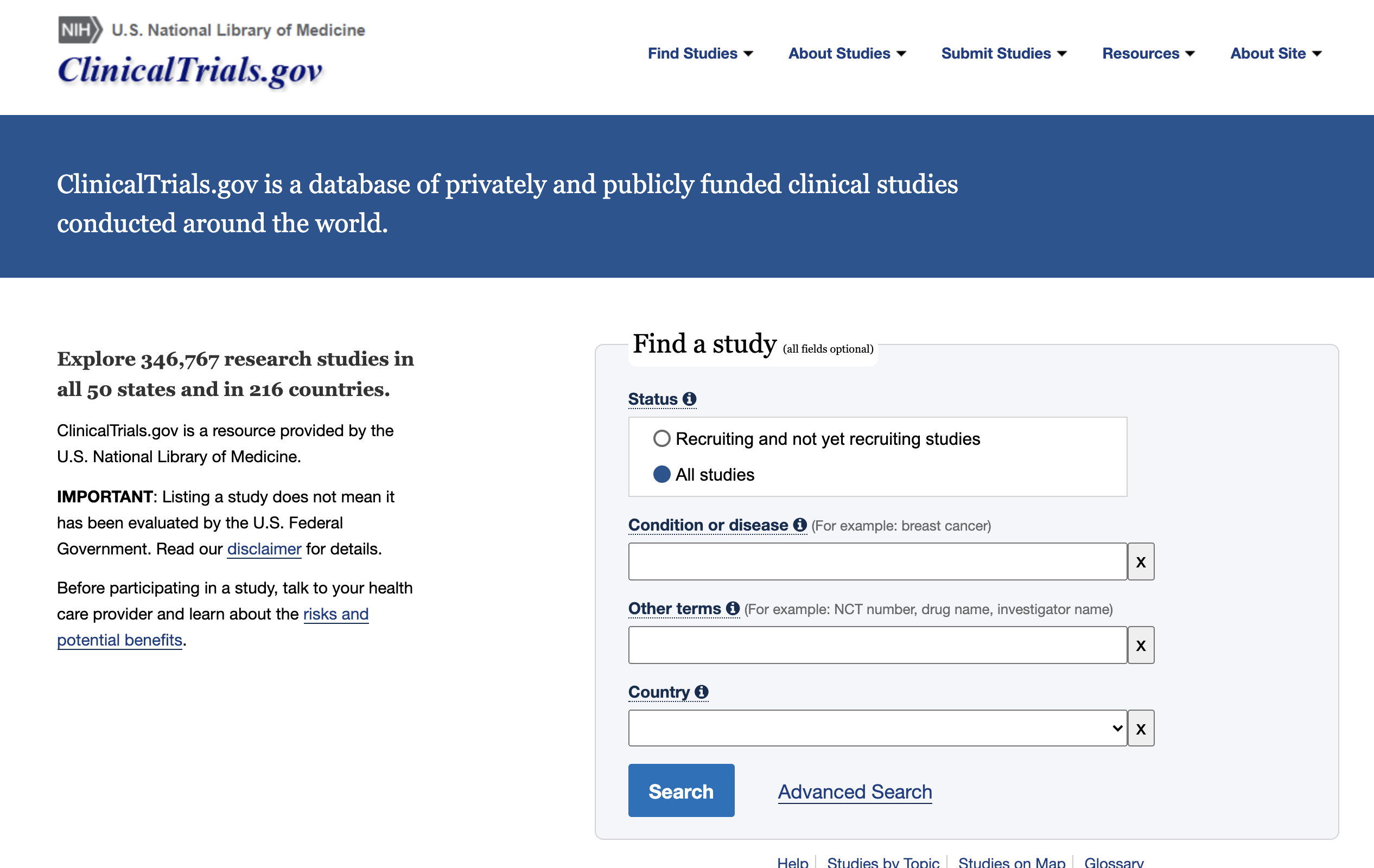
Create your query
ClinicalTrials.gov covers a wide range of clinical trials that occur every day. You can either search a single keyword or create an advanced query with certain study types, locations, ages…

You can find more information about how to use the ClinicalTrials.gov search.
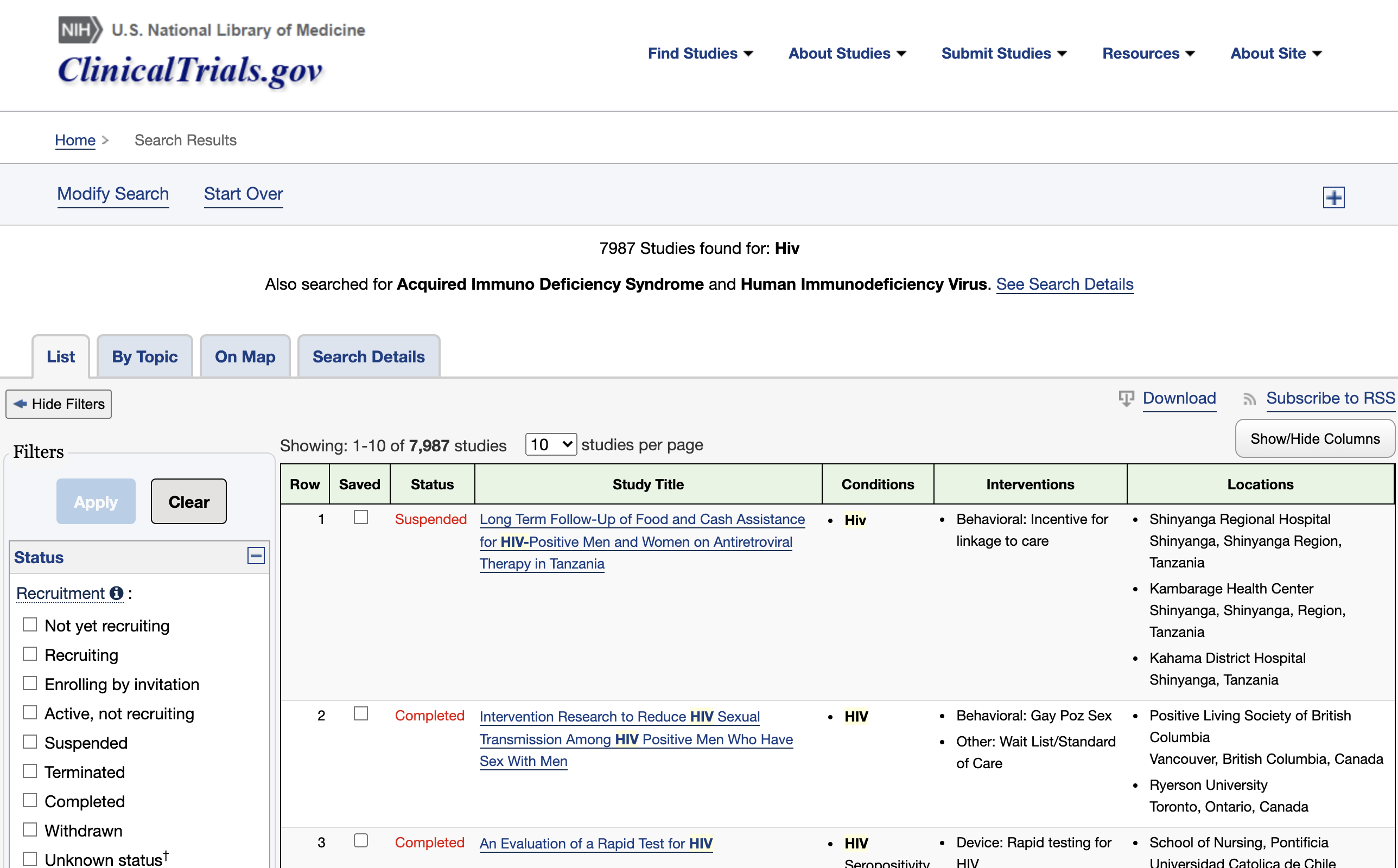
Subscribe to your custom CT.gov RSS feed
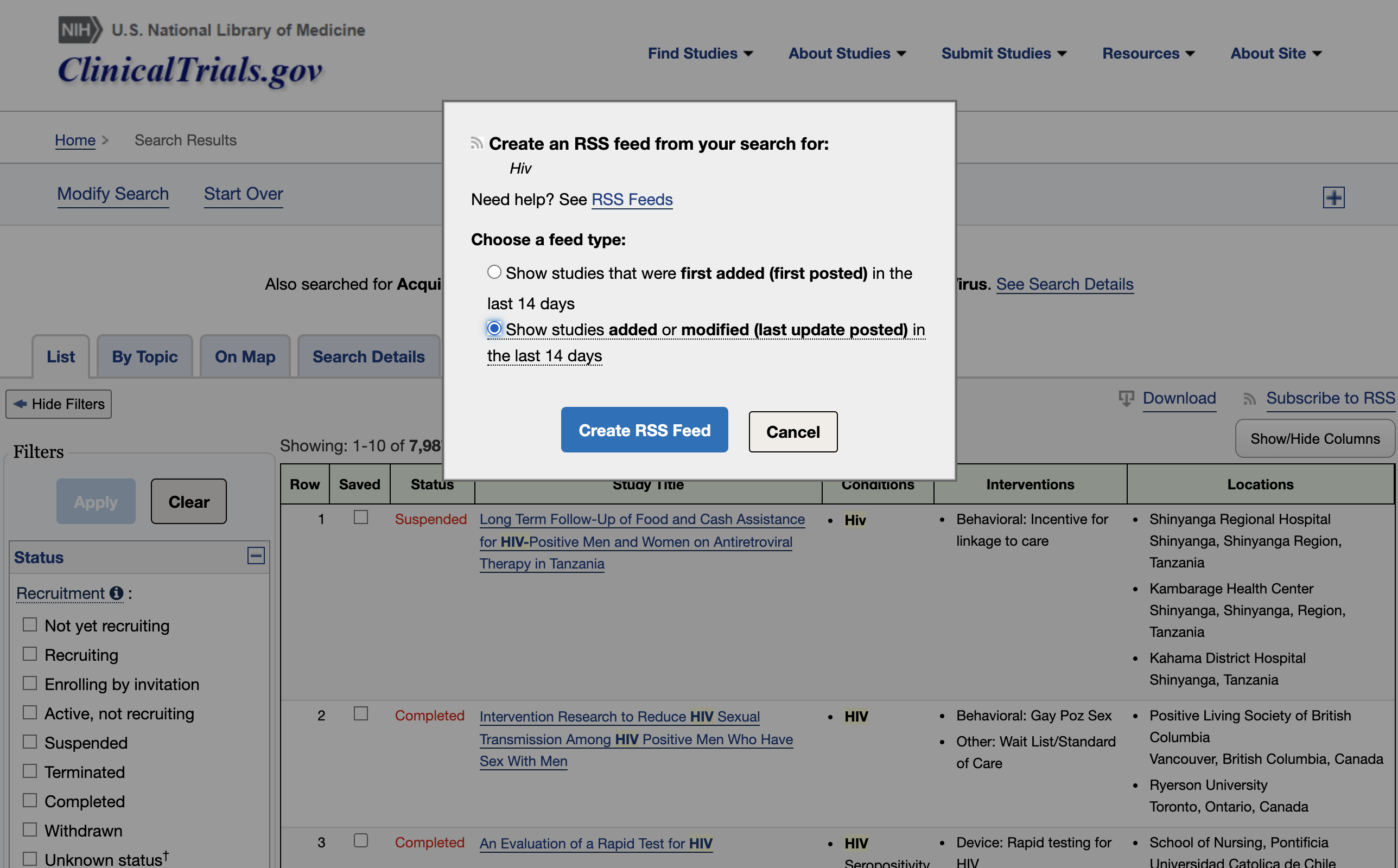
You can look into the results of your query and add additional filters if needed. When you are satisfied with the entries, click ‘Subscribe to RSS’.
This will lead you to the RSS feed you’ll have to copy.
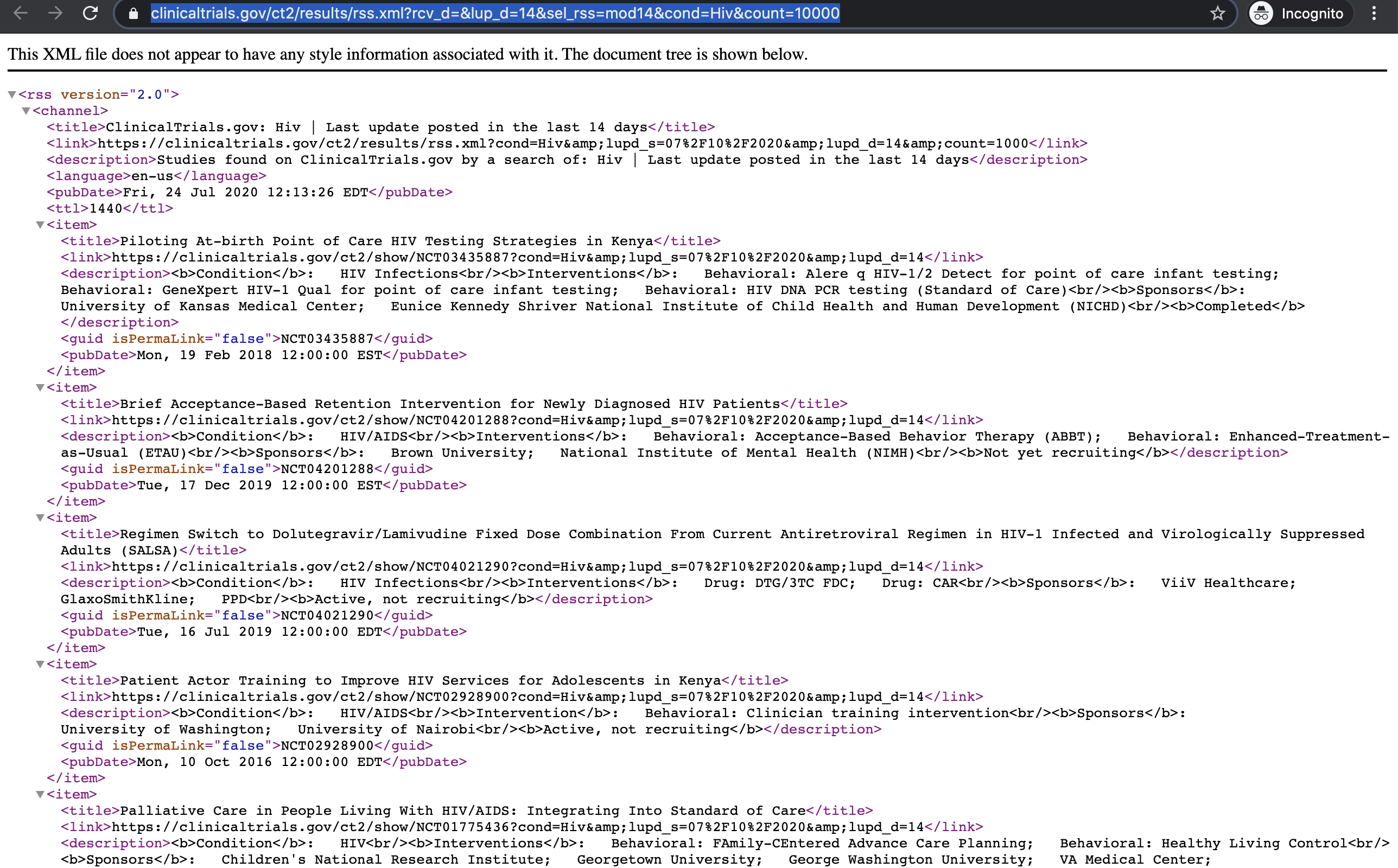
Add your custom CT.gov RSS feed to your Feedly
Click ‘+’ on Feedly to paste the CT.gov RSS feed you just copied. Add it to any of the feeds you’d like and start reading your selected ClinicalTrials.gov content!

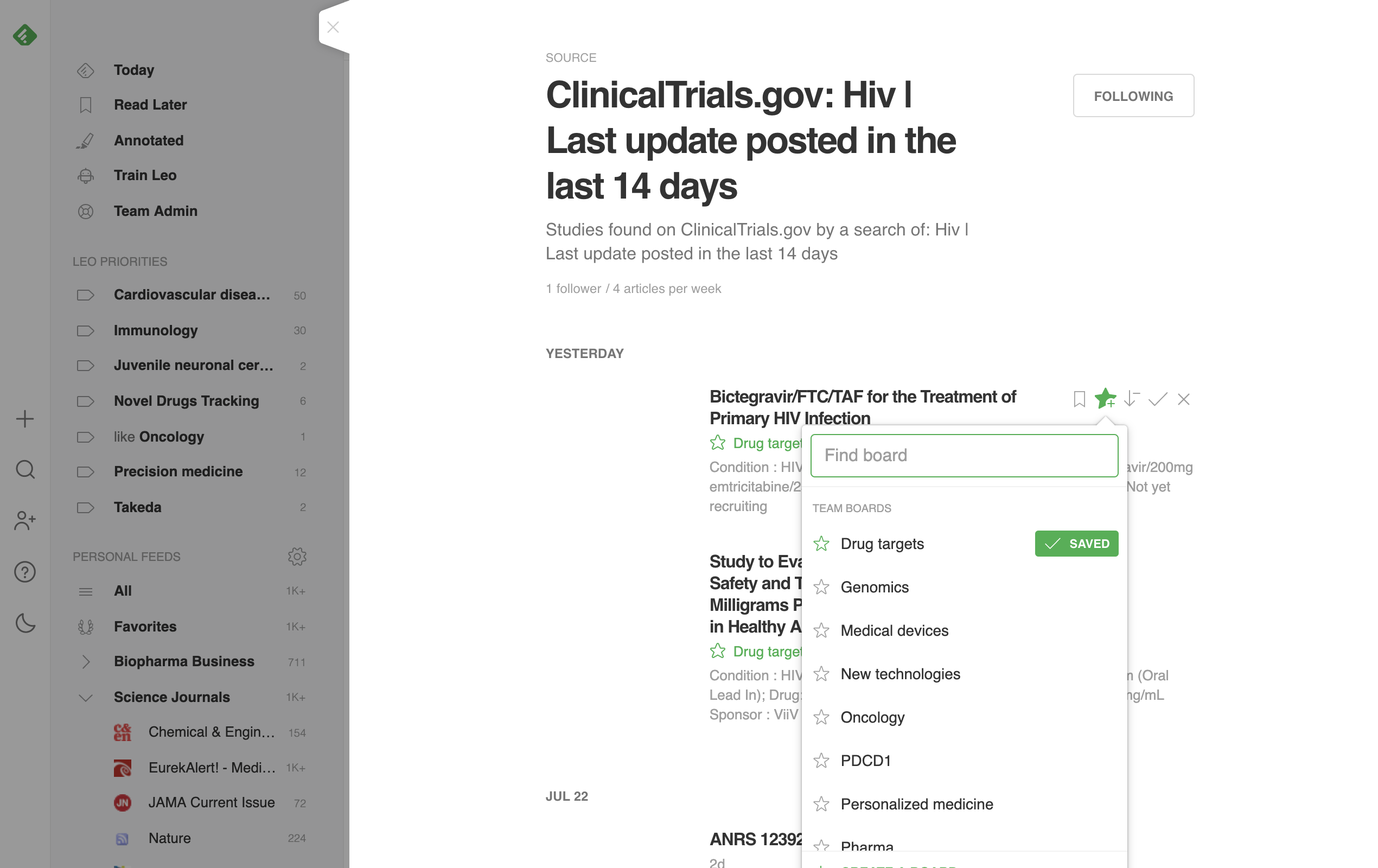
Streamline your biopharma intelligence
We’re excited to see how your team will declutter your feeds and dig deeper into the biopharma news that matter to you. Sign up today and discover Feedly for Biopharma.
If you’re interested in learning more about the Feedly for Biopharma roadmap, you can demo a call by clicking on the button above. 2020 will be a thrilling year with new skills and bold experiments!
[ad_2]
Source link